PIPEX is a collection of commands, scripts and utilities featuring a flexible pipeline to transform the CODEX's resulting images into valuable analysis items. The available options offer a customizable cell segmentation (based on nuclei but with the possibility to refine it through a membrane marker), the automatic generation of a complete FCS CSV file and a configurable and extensive downstream analysis.
- Scripted cell segmentation
- Based on Stardist DAPI nuclei segmentation, configurable cell expansion and optional membrane refinement if a marker is provided.
- Resulting cell segmentation saved as a binary labelled numpy array. All intermediate steps stored as light weighted JPG images for quality control.
- Automatic generation of FCS file
- Direct calculation of segmented cells data and the desired markers intensities.
- If downstream analysis is also performed, additional information (like clustering, interactions, etc...) will be appended to the file.
- Downstream analysis
- Generates the selected results and plots for all the markers, storing them accordingly.
- Batch mode
- PIPEX can process sequentially different CODEX experiments with different configurations of all the above options
- Written entirely in python language, not tied to any specific program that might change and/or be discontinued in the future. Not even really tied to CODEX, can process any arbitrary set of images in a folder!
- More accurate cell segmentation compared to regular Stardist if a good membrane marker is provided.
- PIPEX's generic results can be easily imported to other more graphical o interactive programs. As examples, PIPEX already includes a script to import the cell segmentation (and clusters, if calculated) to QuPath, a post-filtering option to adapt the mask to BIAS, etc...
- The broad scope of the implemented downstream analysis automatically generates a large amount of labelled plots and data that can provide unexpected insights or be easily discarded otherwise.
- Memory efficient, 10k resolution images can be handled by a 16GB regular machine without GPU. Virtually no size limit in linux through automatic swap memory management.
- Quite fast: a full CODEX run of 20 antibodies with 6k resolution images takes 10min to fully process by PIPEX in 16Gb laptop (4min segmentation | 2min analysis | 1min QuPath conversion | 1min mask post-filtering).
- Currently, any linux distribution with python 3. [Note] a docker image is also provided.
- Enough RAM (and/or SSD hard disk to be used as swap memory in case of linux) to handle the working images' resolution. As a guideline:
- Up to 6k resolution -> 8GB.
- Up to 12k resolution -> 16GB.
- NO GPU required, although it can speed up certain pipeline steps if available.
- Install
python3,pipandvirtualenvin case you don't have them yet - Navigate to your desired working directory
- Example:
cd /home/lab/sandbox
- Example:
- Create a virtual environment:
- Example:
python3 -m venv pipex
- Example:
- Unzip the contents of the
pipex.zipinside your virtual environment directory- Following the example above, you should end having a bunch of files (
pipex.py,pipex_process.py, etc...) in the folder/home/lab/sandbox/pipex
- Following the example above, you should end having a bunch of files (
- Navigate to your virtual environment directory and run it:
- Example:
source bin/activate
- Example:
- Install all requirements through pip:
- Example:
pip install -r requirements.txt
- Example:
- Profit!
[Note] a docker image is also provided. Just remember to configure the environment variable for PIPEX's "work" directory. [Note] if you are installing PIPEX in your own laptop you might need some other packages/libraries. Please install these:
sudo apt-get install python3.11-devsudo apt-get install libvipssudo apt-get install -y python3-opencvsudo apt-get install -y gccsudo apt-get install python3-tk
NOTE: remember that you have to access your PIPEX virtual environment before running any PIPEX script! To do so, navigate to your PIPEX working directory and activate it.
- Example:
cd /home/lab/sandboxsource bin/activate
Source images
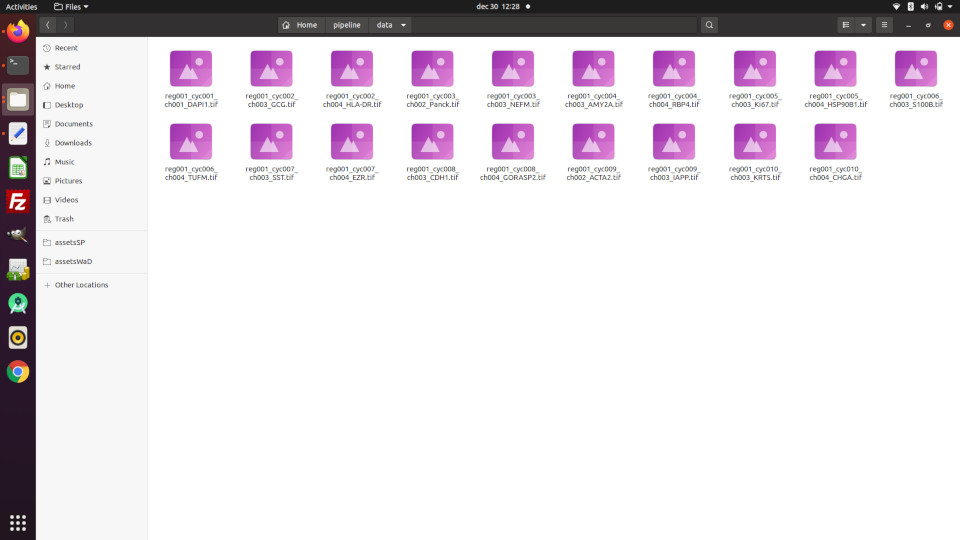
PIPEX requires an accessible folder (referred as "data folder" from now on) containing the images to process and will produce all the results in generated sub-folders. The data folder can contain other files and PIPEX does not modify the original images, but for security and cleanliness sake it's better if you copy the images in a new shiny data folder.
The images in the data folder must reside in the base level (no sub-folders allowed) and must contain the name of the marker (protein) in the filename before the extension.
Example 1: data folder created in {home}/pipeline/data with several images
PIPEX commands
PIPEX runs sequentially a series of commands written in the file pipex_batch_list.txt located in your installation folder. This is a simple txt file, with each command in a single line; you can add blank lines and comments (starting with "#" character) for easy readability.
There are currently available the following commands:
-
run_id <run identifier>: an optional command to number your current PIPEX run with an identifier. Useful if you want to integrate PIPEX in a bigger pipeline, like PyShowwwcase. OBS: PIPEX will assign a random run_id if you don't add this command. -
swap <number of GB>: Linux only, it will generate a temporary swap space in the installation folder with the specified size; the space will be automatically deleted at the end of the full PIPEX process. OBS: it will require root permission/password while executing. -
segmentation.py: performs PIPEX segmentation. Uses the following parameters:-data=</path/to/images/folder>: example -> -data=/home/lab/pipeline/data. OBS: this is the data folder-nuclei_marker=<name before . in image file>: example, from image filename "reg001_cyc001_ch001_DAPI.tif"-> -nuclei_marker=DAPI-nuclei_diameter=<number of pixels>: example -> -nuclei_diameter=20. OBS: this is an approximate guideline, Stardist is smart enough to adapt around 50% above and below.-nuclei_expansion=<number of pixels, can be 0>: example -> -nuclei_expansion=20. OBS: the algorithm is smart enough to not overlap the expansions-nuclei_definition=<optional, gradation between 0.001 and 0.999>: example -> -nuclei_definition=0.5. OBS: this instructs to Stardist algorithm to be less or more strict in its detections. Leave it empty or at 0 for default Stardist model value. Also, if you are using big images and PIPEX is downscaling them, use a low value (i.e 0.1) to help Stardist work better with smaller nuclei.-nuclei_closeness=<optional, gradation between 0.001 and 0.999>: example -> -nuclei_closeness=0.5. OBS: this instructs to Stardist algorithm to allow less or more close (even overlaping at high values) detections. Leave it empty or at 0 for default Stardist model value.-nuclei_area_limit=<optional, number of surface pixels>: example -> -nuclei_area_limit=1600. OBS: this will remove any Stardist nuclei detections with a pixel area larger than the value indicated (and this is done BEFORE nuclei expansion AND membrane watershed). This can be useful to remove wrongly spot detections and/or to let the membrane segmentation kick in with the parameter -membrane_keep.-membrane_marker=<optional, name before . in image file>: example, from image filename "reg001_cyc008_ch003_CDH1.tif" -> -membrane_marker=CDH1. OBS: if you don't use membrane marker you will obtain a basic Stardist segmentation with the specified nuclei expansion-membrane_diameter=<optional, number of pixels>: example -> -membrane_diameter=25. OBS: required if membrane marker is used, this is an approximate guideline, the custom watershed segmentation is smart enough to adapt.-membrane_compactness=<optional, "squareness" of the membrane, gradation between 0.001 and 0.999>: example -> -membrane_compactness=0.5. OBS: required if membrane marker is used, this instructs the watershed algorithm to try to keep a more or less (bigger value is more) square-type segmentation.-membrane_keep=<yes or no to keep segmented membranes without nuclei>: example -> -membrane_keep=no. OBS: this will keep detected membrane segmentations without an underlying nuclei; useful for nuclei shapes not properly segmented by Stardist-adjust_images=<yes or no to enhance poor images>: example -> -adjust_images=yes. OBS: this preprocess nuclei (and membrane if selected) images with poor contrast and/or intensity levels. Does not modify the original images and you can see the altered ones in thequality\_controlsub-folder-custom_segmentation=<optional, file path to a pre-made custom segmentation>: example -> -custom_segmentation=/data/custom_seg.npy. OBS: accepts segmentations in numpy array or image mask file formats. This ignores all the previous segmentation parameters, using this custom segmentation for measuring the markers. Understand that many generated output files will not exists-measure_markers=<list of markers names before . in image files>: example -> measure_markers=DAPI,CDH1,AMY2A,SST,GORASP2.
-
analysis.py: performs PIPEX broad scope analysis. MUST be run AFTER a segmentation. Uses the following parameters:-data=</path/to/images/folder>: example -> -data=/home/lab/pipeline/data. OBS: this is the data folder-image_size=<optional, one-side approximate resolution>: example -> -image_size=1000. OBS: this refers to the resulting plot images' approximate resolution in pixels-analysis_markers=<optional, list of present specific markers to analyze>: example -> -analysis_markers=AMY2A,SST,GORASP2. OBS: if this is not set, the analysis will use all present markers-cellsize_max=<optional, percentage of biggest cells to remove>: example -> -cellsize_max=5. OBS: this refers to the percentge of the biggest cells to be remove for all analysis results-cellsize_min=<optional, percentage of smallest cells to remove>: example -> -cellsize_min=5. OBS: this refers to the percentge of the smallest cells to be remove for all analysis results-custom_filter=<optional, yes or no to apply custom Cell Profiling lab's biomarkers filtering>: example -> -custom_filter=yes. OBS: this will filter known biomarkers following Cell Profiling lab common tweaks.-log_norm=<optional, yes or no to apply log n + 1 normalization>: example -> -log_norm=yes. OBS: this will apply a log1p normalization to the markers intensities-std_norm=<optional, yes or no to apply 0 to 1 re-scale normalization>: example -> -std_norm=yes. OBS: this will apply a standard normalization to the markers intensities-batch_corr=<optional, name of the column in cell_data.csv to perform batch correction by>: example -> -batch_corr=batch_id. OBS: this is the name of the column in the cell_data.csv that differentiates each experiment batch, so they can be separated to perform ComBat batch correction.-use_bin=<optional, yes or no to use binarized columns for clustering>: example -> -use_bin=yes. OBS: this will use the associated binarized columns for each stated marker ([name of the marker] + '_bin_thres') to calculate the selected clustering methods.-quantile_norm=<optional, yes or no to apply quantile normalization>: example -> -quantile_norm=yes. OBS: this will apply an additional quantile normalization to the markers intensities-leiden=<optional, yes or no to perform leiden clustering>: example -> -leiden=yes. OBS: this will perform a leiden clustering and all its associated data and plots-kmeans=<optional, yes or no to perform kmeans clustering>: example -> -kmeans=yes. OBS: this will perform a kmeans clustering and all its associated data and plots.-elbow=<optional, yes or no to show elbow analysis for kmeans>: example -> -elbow=yes. OBS: if kmeans is activated, this will perform a series of 20 kmeans clustering to display the resulting distortion and inertia plots.-k_clusters=<optional, force k number of cluster in kmeans>: example -> -k_clusters=10. OBS: if kmeans is activated, this will use the specified number of k clusters. The default is 10.-refine_clusters=<optional, yes or no to refine cluster results>: example -> -refine_clusters=yes. OBS: this will perform a cluster refinement over leiden and/or kmeans unsupervised results based on the rules described in [cell_types.csv]-neigh_cluster_id=<optional, cluster column name to base the neighborhood analysis on>: example -> -neigh_cluster_id=kmeans_ref. OBS: this will perform a neighborhood analysis based on the specified calculated cluster column
-
generate_geojson.py: generates a GEOjson file to be imported in QuPath. MUST be run AFTER a segmentation and optionally after an analysis (if you want the default clustering). Uses the following parameters:-data=</path/to/images/folder>: example -> -data=/home/lab/pipeline/data. OBS: this is the data folder-included_markers=<optional, list of present specific markers to inlcude>: example -> -included_markers=AMY2A,SST,GORASP2. OBS*: if this is not set, the process will try to use all present markers-cluster_id=<optional, name of the column to add as cluster id information from cell_data.csv>: example -> -cluster_id=kmeans. OBS: this uses the selected column of the cell_data.csv to add clustering data to QuPath detections.-cluster_color=<optional, name of the column to add as cluster color information from cell_data.csv>: example -> -cluster_color=kmeans_color. OBS: this uses the selected column of the cell_data.csv to add clustering data to QuPath detections.
-
generate_filtered_masks.py: can filter and/or tile the generated segmentation mask. It filters the cells present in the postprocessed mask by an arbritary column/values in the cell_data.csv file and/or cuts it in tiles of the specified size. Uses the following parameters:-data=</path/to/images/folder>: example -> -data=/home/lab/pipeline/data. OBS: this is the data folder-field=<optional, name of the column in cell_data.csv to filter the cells by>: example -> -field=cluster_id. OBS: this is the name of the column in the cell_data.csv for the mask to be filtered by-values=<optional, values, comma-separated, present in the selected colum of cell_data.csv to filter the cells by>: example -> -values=3,6,7. OBS: if you create your custom column in the csv file you can filter the segementation by any logic.-tile_size=<optional, number of pixels of each square tile segmented>: example -> -tile_size=2048. OBS: the tiles will always have a square shape (but see-extendoption)-tile_overlap=<optional, number of pixels of surrounding overlap of each square tile segmented>: example -> -tile_overlap=128. OBS: this is a fix pixel size option as oposed as-tile_percentage-tile_percentage_overlap=<optional, tile size\'s percentage of surrounding overlap of each square tile segmented>: example -> -tile_percentage_overlap=10. OBS: this is a pixel percentage size option as oposed as-tile_overlap-tile_relabel=<yes or no to relabel sequentially the tile segments>: example -> -tile_relabel=no. OBS: special option to have the segments of each tile relabeled sequentially, which may be useful to have possible unique identifiers per tile-extend_tile=<yes or no to have bigger border tiles>: example -> -extend_tile=no. OBS: special option to have the last right and bottom border tile of the image extended to reach their limit if the image length and width does not match an exact tile size multiplier
-
preprocessing.py: [BETA] preprocesses the images to try to fix common microscope acquisition problems. It can remove lower and/or upper intensities (background and/or artifacts), increase the exposure and/or generate a heat map. If the image suffers from severe tiling, preprocessing can try to help in different ways: reducing light gradients in each tile, homogenize the intensities of all the tiles and/or smooth the tile cuts. [NOTE] if more than one image is provided in the data folder, preprocessing will calculate all transforming operations in the first file and apply them equally in the rest of them; this is useful to modify proportionally all markers/channels present in the same original microscope image, which will most probably suffer of the same light/intensity problems.-data=</path/to/images/folder>: example -> -data=/home/lab/pipeline/data. OBS: this is the data folder-threshold_min=<number, percentage of intensity>: example -> -threshold_min=1. OBS: this is minimum percentage of the global intensity (the image limit) from which all intensities below will be deleted.-threshold_max=<number, percentage of intensity>: example -> -threshold_min=99. OBS: this is maximum percentage of the global intensity (the image limit) from which all intensities above will be deleted.-balance_tiles=<yes or no>: example -> -balance_tiles=yes. OBS: this activates the option to try to homogenize the intensities of all the tiles-tile_size=<number of pixels>: example -> -tile_size=1844. OBS: this is the size of the image square tiles-bright_levels=<number, main levels of intensity in the image [normally 3-5 and their 2 selected focus]>: example -> -bright_levels=4:1:3. OBS: this is the number of otsu thresholds to use while processing the image and the two ones selected as main intervals. If you use 0 it will default to 3:1:2 but provide a sample of many otsu intervals as output files.-light_gradient=<number, factor of complexity of light issues [1 to 4 should be enough]>: example -> -light_gradient=3. OBS: this is the exponential value of squared sub-tiles that will be used to try to reduce the light gradients in each tile.-stitch_size=<number of pixels>: example -> -stitch_size=20. OBS: this specifies the width of the region to use while performing the smooth operation in the tile cuts-exposure=<number, percentage of the base intensity>: example -> -exposure=150. OBS: this increases the exposure of the image once it has been preprocessed-heat_map=<yes or no>: example -> -heat_map=yes. OBS: this generates a heat_map of the preprocessed image
-
dot_extraction.py: [BETA] extract dots using BigFish library.-data=</path/to/images/folder>: example -> -data=/home/lab/pipeline/data. OBS: this is the data folder-marker=<name before . in image file>: example, from image filename "reg001_cyc001_ch001_DO.tif"-> -marker=DO-voxel_size=<optional, size of the voxel in nm>: example -> -voxel_size=103. OBS: this is the size of the voxel in nm-spot_radius=<optional, radius of the spot in nm>: example -> -spot_radius=150. OBS: this is the radius of the spot in nm-cluster_radius=<optional, radius of the cluster in nm>: example -> -cluster_radius=350. OBS: this is the radius of the cluster in nm-cluster_nb_min_spots=<optional, minimum number of spots in a cluster>: example -> -cluster_nb_min_spots=4. OBS: this is the minimum number of spots in a cluster-dense_alpha=<optional, alpha parameter for dense region decomposition>: example -> -dense_alpha=0.7. OBS: this is the alpha parameter for dense region decomposition-dense_beta=<optional, beta parameter for dense region decomposition>: example -> -dense_beta=1. OBS: this is the beta parameter for dense region decomposition-dense_gamma=<optional, gamma parameter for dense region decomposition>: example -> -dense_gamma=5. OBS: this is the gamma parameter for dense region decomposition
-
generate_tissuumaps.py: generates TissUUmaps project for interactive visualization and quality control of the downstream analysis and cell segmentation. Uses the following parameters:-data=</path/to/images/folder>: example -> -data=/home/lab/pipeline/data. OBS: this is the data folder-include_marker_images=<yes or no or list of present specific markers to display as image layers>: example -> -include_marker_images=DAPI,SST,GORASP2. OBS: this includes the specified markers as image layers in the TissUUmaps project. If this is not set, no image layers will be included-include_geojson=<yes or no to include cell segmentation as regions>: example -> -include_geojson=yes. OBS: this includes the cell segmentation as regions in the TissUUmaps project. If this is not set, no regions will be included-compress_geojson=<yes or no to compress geojson regions into pbf>: example -> -compress_geojson=yes. OBS: this includes the cell segmentation regions as a compressed pbf file-include_html=<yes or no to export html page for sharing the TissUUmaps project on the web>: example -> -include_marker_images=yes. OBS: this includes the html page for sharing the TissUUmaps project on the web. A web server is needed to visualize the exported web page
Example 2: contents of pipex_batch_list.txt for the images from example 1
#my ubuntu laptop needs 8GB more of RAM to handle the images
swap 8
#running ESPACE experiment 3
segmentation.py -data=/home/lab/pipeline/data -nuclei_marker=DAPI -nuclei_diameter=20 -nuclei_expansion=20 -membrane_marker=CDH1 -membrane_diameter=25 -adjust_images=yes -measure_markers=DAPI,CDH1,HLA-DR,CHGA,KRT5,IAPP,ACTA2,GORASP2,EZR,SST,TUFM,S100B,HSP90B1,Ki67,RBP4,AMY2A,NEFM,Panck,HLA-DR,GCG
analysis.py -data=/home/lab/pipeline/data -image_size=1000 -leiden=yes
#need full QuPath integration, with cluster
generate_geojson.py -data=/home/lab/pipeline/data -expand=yes
dot_extraction.py -data=/home/lab/pipeline/ -marker=DO -voxel_size=103 -spot_radius=150 -cluster_radius=350 -cluster_nb_min_spots=4 -dense_alpha=0.7 -dense_beta=1 -dense_gamma=5
generate_tissuumaps.py -data=/home/lab/pipeline/data -include_marker_images=DAPI,CDH1,HLA-DR,CHGA,KRT5 -include_geojson=yes -compress_geojson -include_html=yes
Run the script
Once you have written in pipex_batch_list.txt the commands to be sequentially executed by PIPEX you just need to run the following command from the command line (OBS: you have to run this command from PIPEX's installation folder!):
python3 pipex.py
This will generate the following sub-folders and items inside the data folder:
analysisfolder: it contains the following items:segmentation_data.npyfile: the labelled cell regions in numpy array format (for further computing analysis)segmentation_data_filtered.npyfile: the filtered labelled cell regions in numpy array format (if you have executed the generate_filtered_masks step)segmentation_data_filtered_tile_X_X.npyfile: each tile of the filtered labelled cell regions in numpy array format (if you have executed the generate_filtered_masks step with tiling)segmentation_binary_mask.tiffile: the cell segmentation binary mask in TIFF format.segmentation_mask_filtered.tiffile: the filtered cell segmentation mask in TIFF format (if you have executed the generate_filtered_masks)segmentation_mask_filtered_tile_X_X.tiffile: each tile of the filtered cell segmentation mask in TIFF format (if you have executed the generate_filtered_masks step with tiling)segmentation_mask_show.jpgfile: the cell segmentation mask over the first image (usually DAPI) in JPG format.cell_data.csvfile: the Flow Cytometry Standard file (as CSV) with cell segmentation metadata and all markers intensities calculated (and clustering if analysis command has been performed).cell_segmentation_geo.jsonfile: the GEOjson file that can be imported in QuPath as cell segmentation (and clustering if analysis command has been performed). OBS this file will only be present if you have run the generate_geojson command)
analysis/downstreamfolder: it contains the following items:- Several image files: the filename shows the data represented in them. OBS this files will only be present if you have run the analysis command)
cell_data_norm.csv: filtered and normalized version ofcell_data.csvgeneral file. OBS this file will only be present if you have run the analysis command)cell_data_markers.csv: aggregated data for each of the analyzed markers. OBS this file will only be present if you have run the analysis command)anndata.h5ad: AnnData object containing the analysis output. OBS this file will only be present if you have run the analysis command)anndata_TissUUmaps.h5ad: TissUUmaps project file. OBS this file will only be present if you have run the generate_tissuumaps command)
analysis/quality_controlfolder: it contains the following items:- Several image files: these are image representations of intermediate steps of the cell segmentation process. Useful as post-verification and/or to refine the parameters if the result is not what you expected.
preprocessedfolder: it contains the following items:- Same image file: the preprocessed resulting image.
- Several image files: these are other image generated by the preprocessing step, including heat maps.
Implicit batch mode
Remember that nothing prevents you to run several different experiments by using different data folders in the command list introduced in pipex_batch_list.txt!
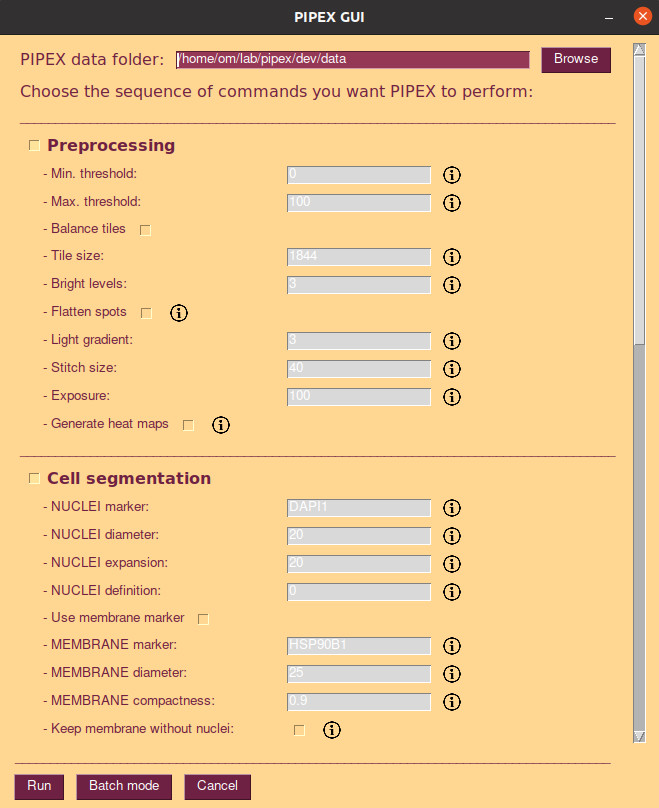
PIPEX offer a simple and easy GUI to run single experiments. It does not offer multiple data folders configurations, but it's flexible enough to configure your pipeline with all available steps and parameters. To open the GUI just type in your PIPEX's virtual environment:
sh pipex.sh
NOTE: remember that you have to access your PIPEX virtual environment before running any PIPEX script! To do so, navigate to your PIPEX working directory and activate it.
- Example:
cd /home/lab/sandboxsource bin/activate
If you add the generate_geojson command to PIPEX command list a cell_segmentation_geo.json file will be generated in your analysis sub-folder. You can import this file in QuPath via scripting. To do so:
- Create your regular QuPath project (import images, etc...)
- Open QuPath's script editor.
- Copy the contents of
qupath_import_example.groovyfile available in your PIPEX installation folder. OBS: you can of course create your own script to handle the GEOjson file generated by PIPEX. - Change the folder path written in the script for the location of the
cell_segmentation_geo.jsonyou want to import. - Run the script.
- Enjoy!
NOTE: the geojson structure and groovy script provided work for current QuPath 0.3 version. QuPath program is in a early stage of development, with a very lacking in-depth (scripting wise) documentation. Most of the geojson format and importing mechanism has been deduced from the source code and nothing prevents its author to change them at any point in the future.
If you add the generate_tissuumaps command to PIPEX command list a anndata_TissUUmaps.h5ad file will be generated in your analysis/downstream sub-folder. You can open this file in TissUUmaps. To do so:
- Install TissUUmaps (https://tissuumaps.github.io/TissUUmaps-docs/docs/intro/installation.html)
- Load the
anndata_TissUUmaps.h5adfile in TissUUmaps
If you add the include_html=yes parameter to the generate_tissuumaps command, a TissUUmaps_webexport folder will be generated in your analysis/downstream sub-folder. You can share this file on a web server, and access it from any web browser.
NOTE: TissUUmaps requires your images to be in TIFF format and be name exactly as your markers (for example: DAPI.tif, CPEP.tif, etc...)
PIPEX can be integrated as a step in a bigger pipeline or queue.
- By default, a random
run_idis assigned to every PIPEX operations batch and a file namedLAST_RUN_IDcontaining the same identifier is generated in the root folder once the process is finished. - You can add a file in the root folder named
run_id.txtcontaining a specific identifier if you want to force PIPEX to use it for the next run. TheLAST_RUN_IDfile will be updated accordingly when the process is finished. - You can also directly specify a run identifier by the PIPEX command
run_id
PIPEX's custom cell segmentation features a combination of different techniques and existing algorithms to try to surpass certain of their individual shortcomings.
-
The basic initial part of the segmentation is performed by Stardist, very fast and accurate for nuclei detections. Nevertheless, Stardist has some limitations as a generalistic cell segmentation mechanism:
- It can only detect convex shapes. This is still a limitation in PIPEX, although most of the cells seems to be compliant.
- It only detects cell nuclei. PIPEX offers the possibility to automatically expand this detection and try to fit it into the region defined by an optional membrane marker.
- With the regular usage proposed in its documentation, Stardist is prone to out of memory errors and can't address big resolution images. PIPEX uses an internal method present in Stardist's source code to reduce the memory usage and an optional swap memory mechanism (only in linux) to dynamically adapt to different images. In addition, for really big images that can't fit your memory allocation, PIPEX downscales them to work and upscales the results seamlessly.
-
After Stardist segmentation, PIPEX (optionally) expands the detections to the required size, taking care to not overlap the neighboring cells.
-
If a membrane marker is designed, PIPEX performs a separate custom watershed segmentation over the membrane image with configurable detection size and compactness.
-
Finally, PIPEX combines both segmentations by:
- Merging all watershed segments that share a Stardist basic (not expanded) nuclei detection
- Confronting the expanded nuclei detections against the segments of the watershed membrane and cutting them to adapt to those.
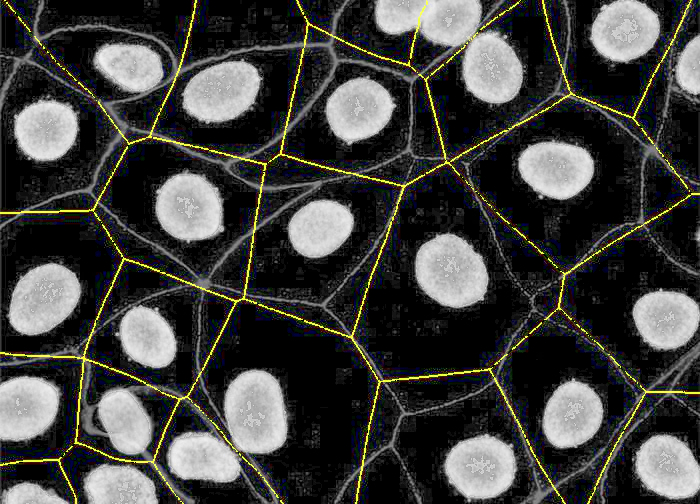
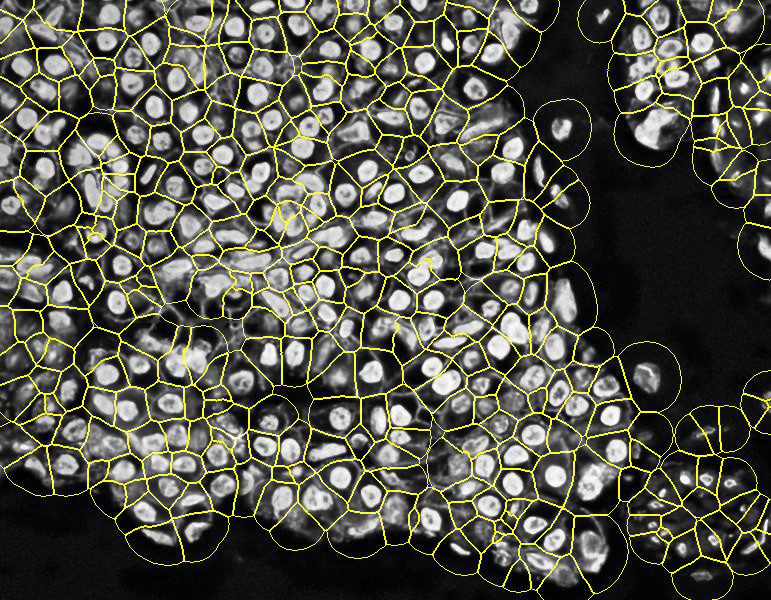
The result is a more realistic cell segmentation than the Stardist + expansion method, very common nowadays. To see the differences, you can compare results over some samples:
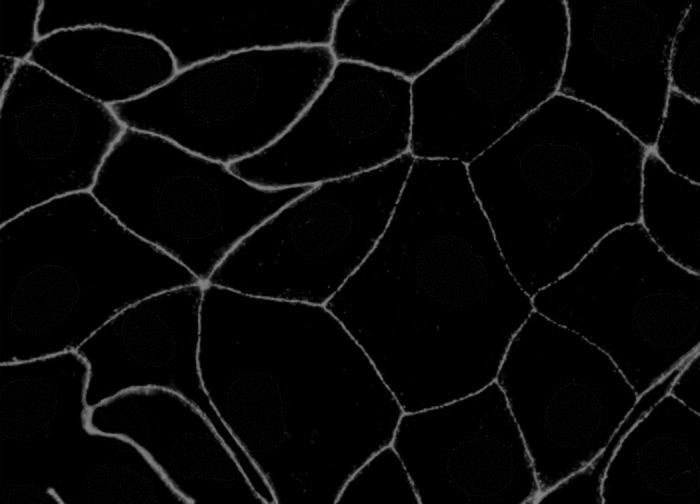
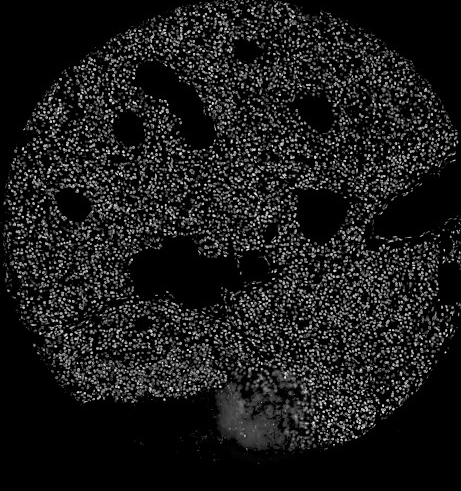
Sample original image 1:
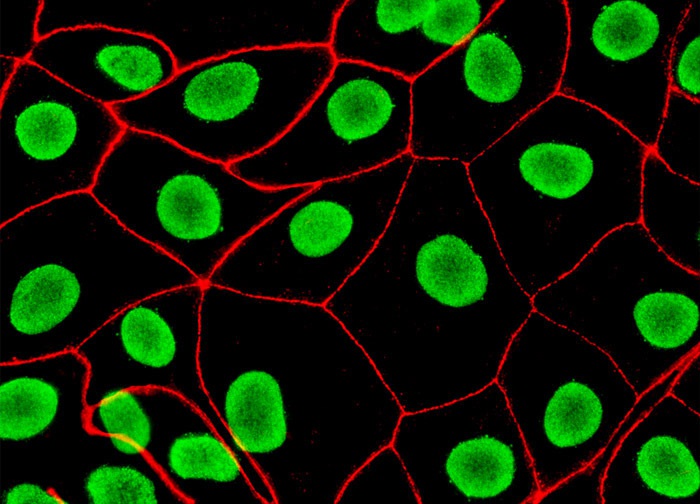
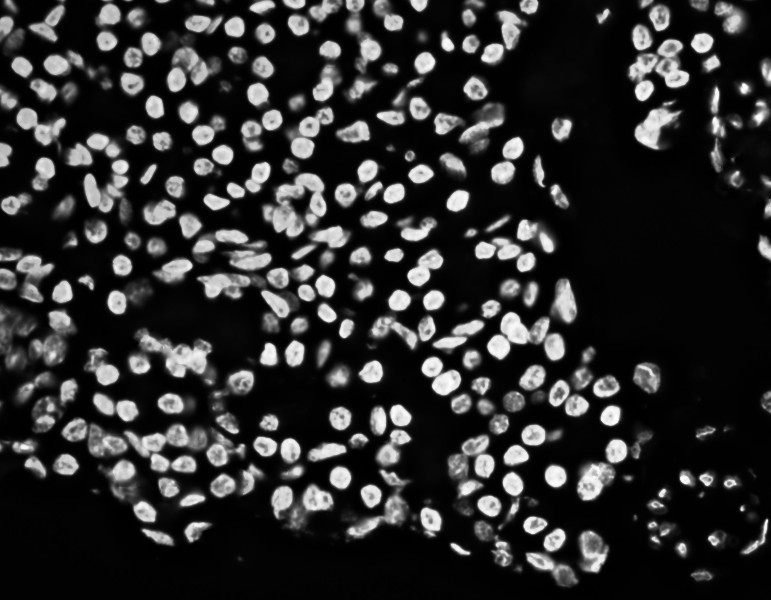
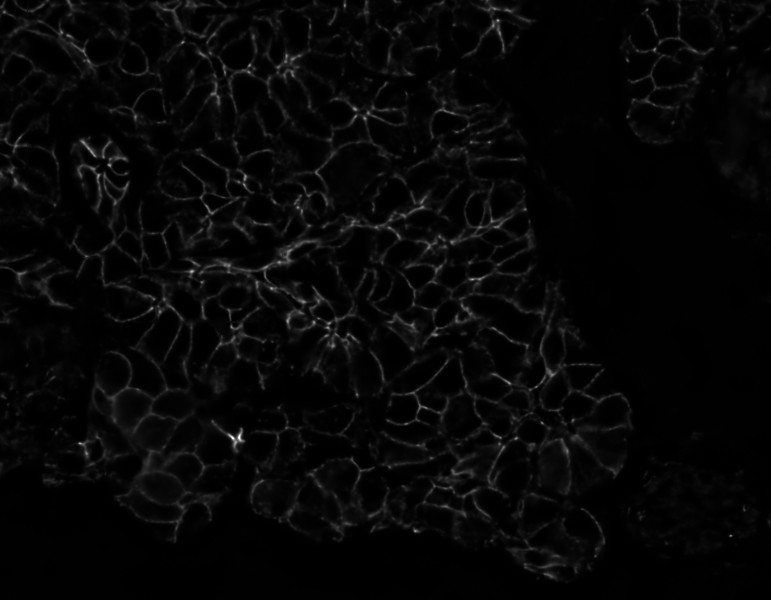
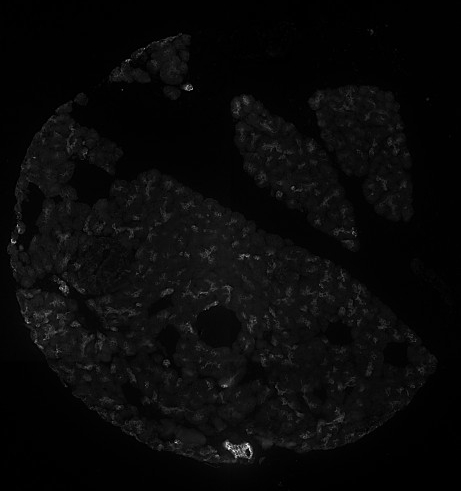
Separated nuclei and membrane channels:
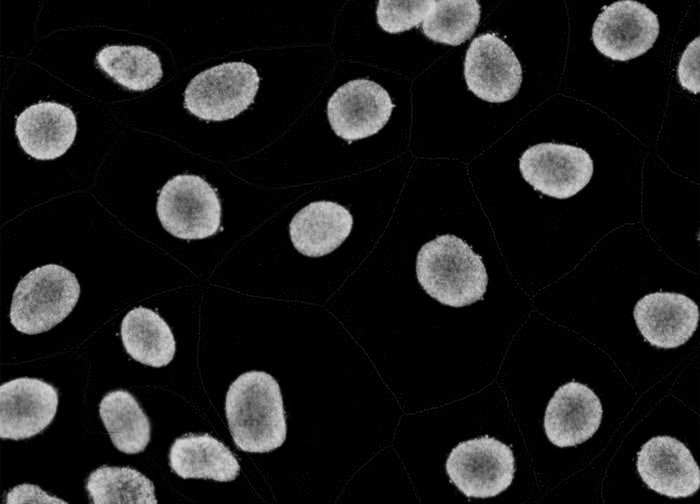
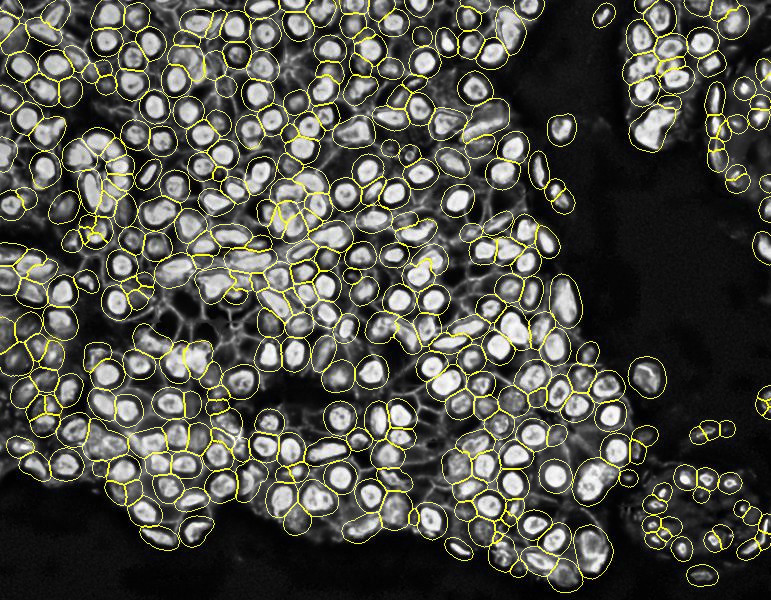
Stardist nuclei segmentation: note that this segmentation would not take into account any kind of markers in each cell cytoplasm and membrane.
Stardist expanded segmentation: note how PIPEX avoid to overlap the detections. Of course, this is better than the basic Stardist result because it will account for some non-nuclei markers, but the calculated cells don't follow the real membrane distribution
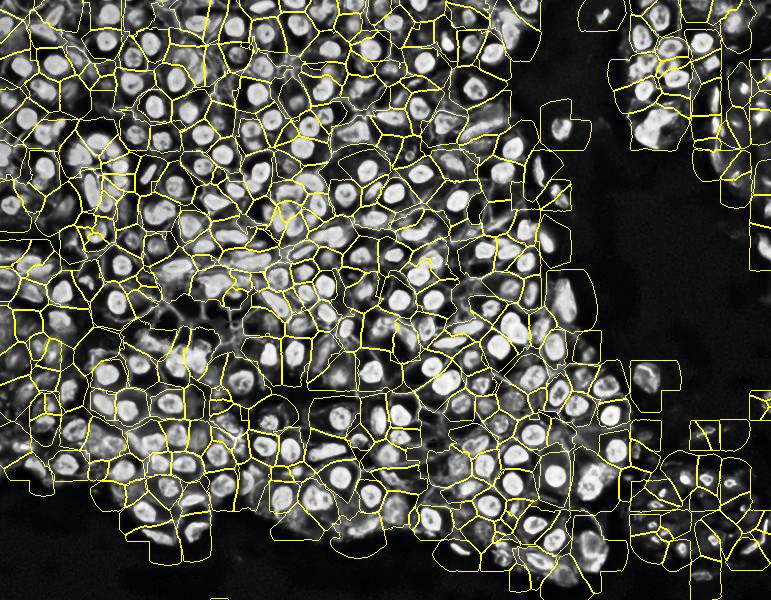
This is the industry's nowadays most common approach. You can find different types of expansions over basic Stardist in many third-party products:
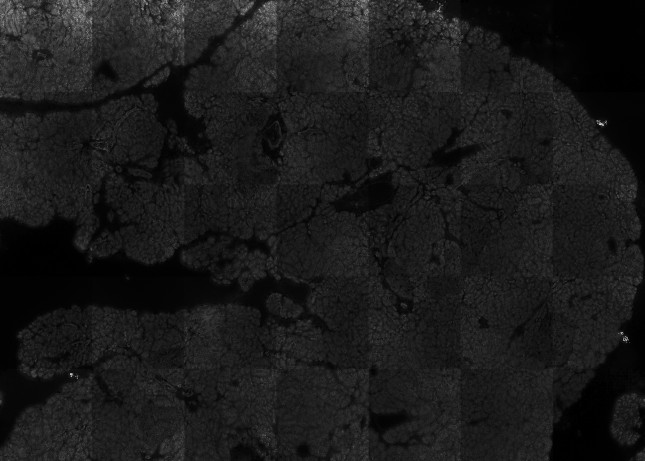
Stardist expanded in QuPath: note how QuPath's expansion overlaps, which will cause a double reading of the markers in the affected cells
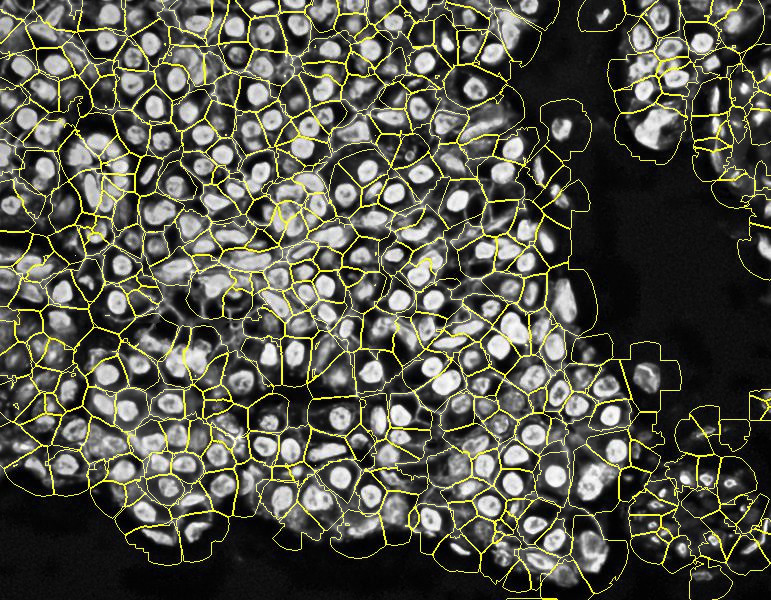
Stardist expanded in Halo AI: note how Halo AI's expansion overlaps (having the same problem as QuPath) and, even worse, fusions different cell nucleis.
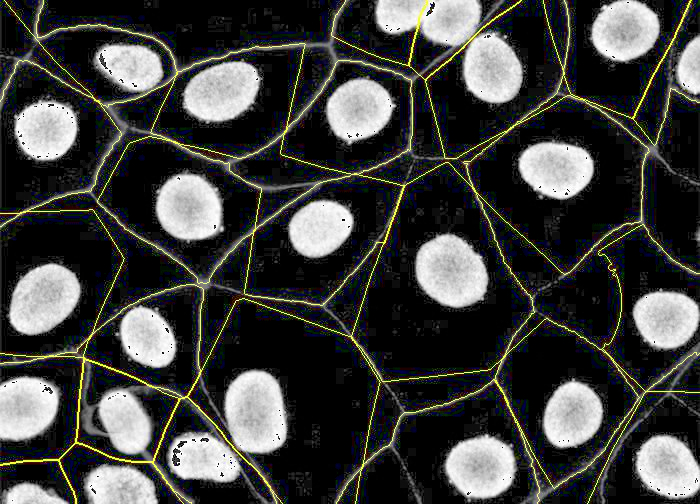
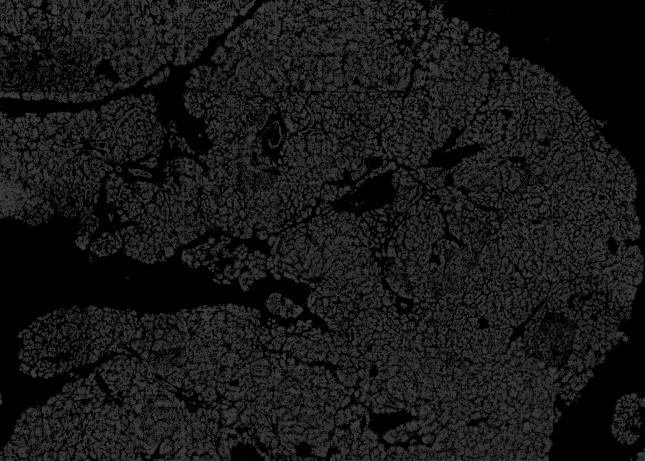
PIPEX final segmentation: by using a membrane marker, PIPEX can correct its stardist expansion to try to fit it into the different membrane segments. As you can see in the image, is far from perfect, but offers a more realistic cell segmentation.
Finally, one of the best features in PIPEX segmentation is its flexibility: if you want just basic stardist, you can stop there and have it your way. You can also add the desired amount of expansion and forget about the membrane marker. You can even duplicate (even easier with linux by creating a symlink) the data folder and run several PIPEX segmentations with different parameters and options!
See different possible outcomes for several parameters over the same sample:
Sample 2 Separated nuclei and membrane channels:
PIPEX basic Stardist, nuclei size 20, expansion size 5 (ignores membrane)
PIPEX basic Stardist, nuclei size 20, expansion size 20 (ignores membrane)
PIPEX basic Stardist, nuclei size 20, expansion size 20, membrane size 30, compactness 0.9
PIPEX basic Stardist, nuclei size 20, expansion size 20, membrane size 30, compactness 0.001
PIPEX offers an optional preprocessing step to try to fix CODEX's (and other multi-tile microscope software) most common imaging issues. Due to the limitations of its close code, PIPEX works directly with the stitched tiles in a best effort attempt to alleviate present problems in the final image.
Preprocessing offers, at high level, the following options (in order):
- Threshold intensities removal: select a minimum and/or maximum percentage of absolute intensity to be removed from the image. Useful to clean background and tissue folding or precipitates.
- Tiles fixes:
- Reduce light gradients: subdivides each local tile in kernels and tries to find and eliminate gradients of light causing shades and/directional light focuses.
- Homogenize bright levels: analyzes all tiles, finding the important thresholds in the global image intensities, and tries to homogenize the histogram proportions on each tile, reducing the bright differences between them.
- Stitch smoothing: smooth the area around the tile cuts.
- Exposure: enhance or decrease in percentage the final intensities of the image.
Through a combination of these techniques (which can be easily tried and re-tried with PIPEX's configurable nature), problematic images can be salvaged and/or improved for further analysis. Some examples with their chosen parameters are shown below:
Gradient lights and unbalanced tiles. Preprocessed with -threshold_min=1 -threshold_max=100 -exposure=100 -heat_map=no -tile_size=1844 -bright_levels=3 -flatten_spots=no -light_gradient=3 -balance_tiles=yes -stitch_size=40
Heavy background and unbalanced tiles. Preprocessed with -threshold_min=3 -threshold_max=100 -exposure=100 -heat_map=no -tile_size=1844 -bright_levels=3 -flatten_spots=no -light_gradient=1 -balance_tiles=yes -stitch_size=40
Very damaged image: background signal, shifting gradient lights, very unbalanced tiles, precipitates, etc... Preprocessed with -threshold_min=7 -threshold_max=100 -exposure=100 -heat_map=no -tile_size=1844 -bright_levels=4:1:3 -flatten_spots=yes -light_gradient=3 -balance_tiles=yes -stitch_size=40
There's a full presentation about PIPEX's preprocessing technique available. Just download it and open preprocessing_presentation.html in your browser.
PIPEX's analysis step includes an optional marker filtration commonly used in Cell Profiling lab. It comprises the following (simultaneous) items:
DAPI1% top ranked intensities cell removalCDH11% top ranked intensities cell removalCTNNB11% top ranked intensities cell removal
Please make sure you the name of your marker column is a strict match with the aforementioned ones
PIPEX's analysis step includes the possibility to refine the unsupervised clustering results (leiden and/or kmeans). This can help you with the manual annotation and merging of the clusters automatically discovered.
The idea behind the cluster refinement algorithm is to explore the ranked genes associated to each cluster and try to match them with rules stated by the user. The algorithm then assigns a confidence score per cluster and rule, depending how close its ranked genes are to the rule/s definition/s. Finally, the refinement picks per cluster the annotated cluster with higher confidence (ties are solved by row order).
To use the cluster refinement, you have to create a cell_types.csv file with rows containing the following information:
cell_group: used as a prefix for the manually annotated cluster name. The final cluster name will be[cell_group]-[cell_type]-[cell_subtype]cell_type: used as a interfix for the manually annotated cluster name. The final cluster name will be[cell_group]-[cell_type]-[cell_subtype]cell_subtype: used as a suffix for the manually annotated cluster name. The final cluster name will be[cell_group]-[cell_type]-[cell_subtype]rank_filter: used to direct the refinement procedure to use only certain ranked genes. Default isall(no filtering), you can usepositive_only(use only ranked genes with positive values)min_confidence: by default, the refinement procedure aggresively merges all clusters that minimally fullfil the indicated rules. You can force the process to be more strict by using a highermin_confidenceprobability (values from 0 to 100)marker[n]andrule[n]pairs: you can add an arbritary amount (at least one!) of marker rules to guide the algorithm how to annotate/merge the automatically discovered clusters. The marker value must match one of your analysis markers and the rule states how the marker should be relatively placed amongst the ranked genes (valueshigh,medium,low)
Here's and example of how a cell_types.csv file usually looks:
cell_group,cell_type,cell_subtype,rank_filter,min_confidence,marker1,rule1,marker2,rule2,marker3,rule3
artifact,fold,unknown,all,10,CBS,high,CHGA,high,AMY2B,high
endocrine,islet,all,positive_only,10,CHGA,high,CPEP,high,AMY2B,low
exocrine,acinar,unknown1,all,10,CBS,high,AMY2B,high
endothelial,vessels,all,positive_only,30,CD31,high,aSMA,high
epithelial,ductal,unknown,all,10,KRT19,high,PANCK,high
immune,potential,artifact,all,10,HLADR,high,NPDC1,high,aSMA,low